- Recognized as an Innovative Experimental Solution for Cardiac Safety Evaluation -
Cellames' cutting-edge technology has been included in the "Comprehensive Arrhythmia Assessment Guidelines" published by the Pharmacology and Narcotics Research Division of the National Institute of Food and Drug Safety Evaluation (NIFDS), under the Ministry of Food and Drug Safety (MFDS), South Korea.
This guideline provides essential experimental methods for cardiac toxicity evaluation and new drug development, and Cellames' product has been introduced as a key experimental tool in the evaluation method using stem cell-derived cardiomyocytes.
This inclusion is a testament to the reliability and excellence of Cellames' technology as a trusted cardiac safety evaluation solution. We are proud to contribute to the advancement of the biopharmaceutical and drug safety industries both in Korea and globally.
Moving forward, Cellames will continue to lead innovations in bio-research and pharmaceutical safety evaluation, expanding its influence in the global market.


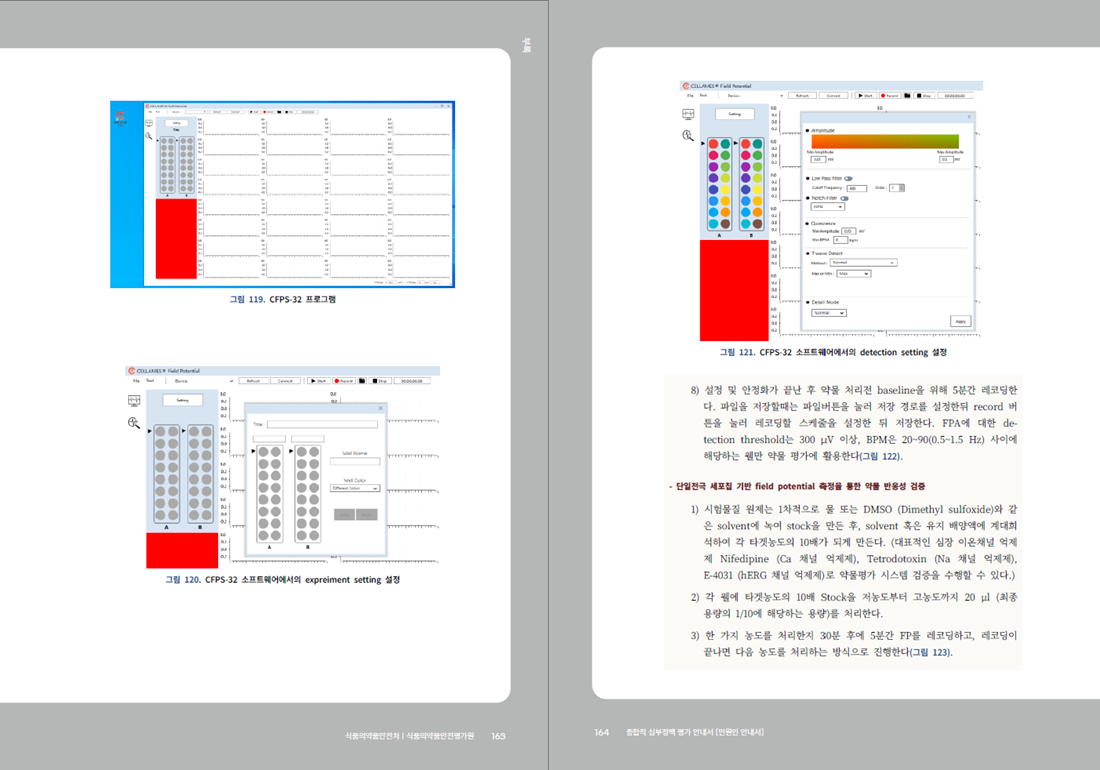
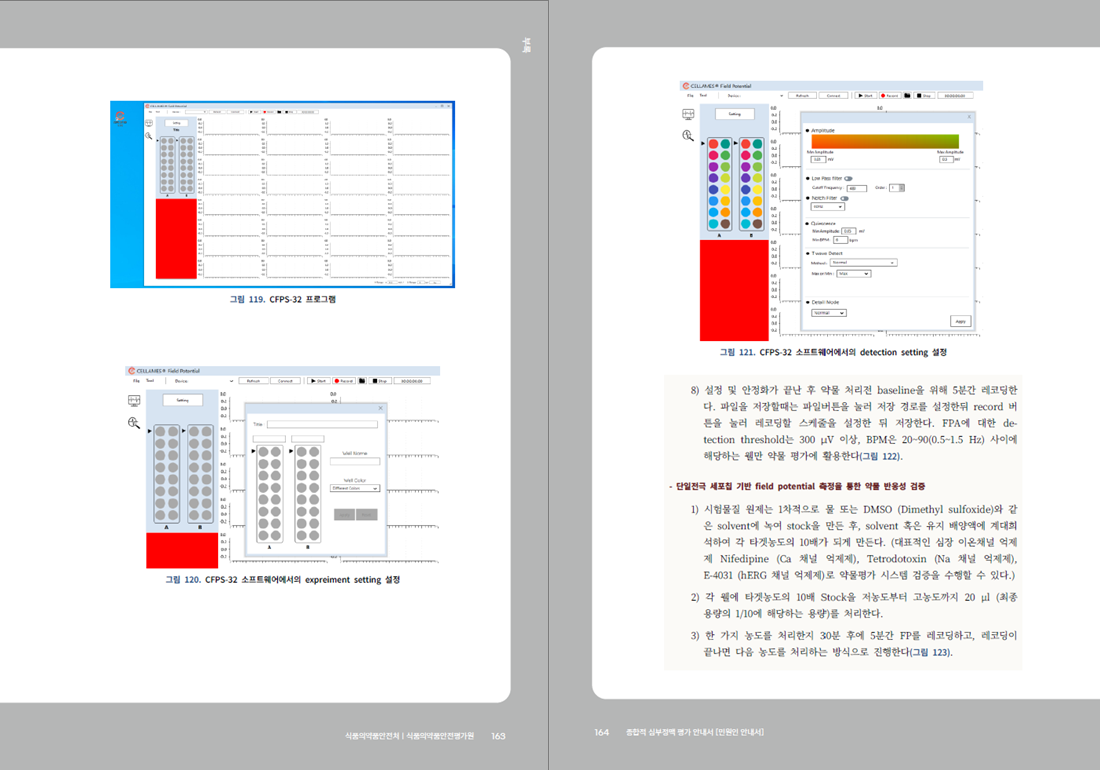
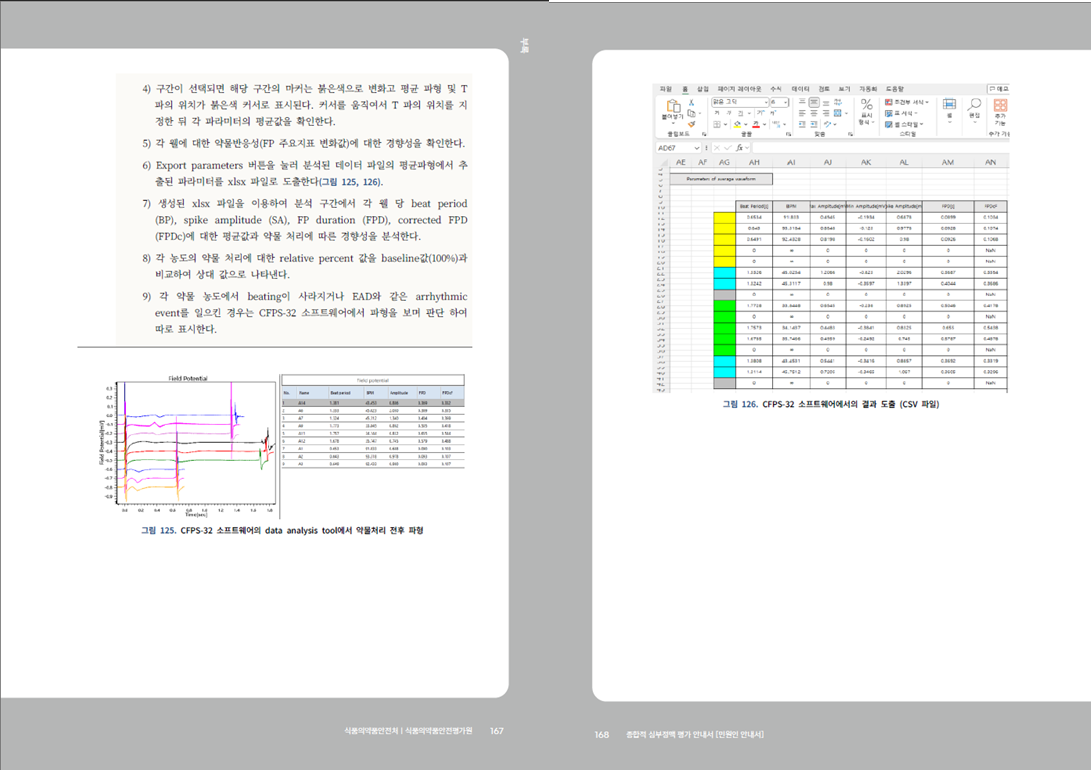
- For more details, please refer to the "Comprehensive Arrhythmia Assessment Guidelines" published by the MFDS Pharmacology and Narcotics Research Division.
- View the full guideline here
- For inquiries regarding our products and research collaborations, please contact the Cellames Customer Support Team.
- Cellames is committed to shaping the future of scientific research together with you!